Genetic code, quantum physics and the 3/2 ratio
Quantum analysis of the atoms constituting the genetic code
Jean-Yves Boulay
Abstract. The analysis of the quantum structure of the five chemical elements composing the coded twenty amino acids and the four coding nucleotides of DNA working in the organization of the genetic code reveals an opposition of their respective constituents in always an arithmetic ratio of value 3/2 according to the parity of the number of their quantum shells. Also, the quantum analysis of the amino acid Glycine, the smallest component of peptides that can be confused with saturated base, reveals the same arithmetic oppositions of 3/2 value of its components by the differentiation, operated according to their number of protons, of its five chemical groups. In sidelines to this study, a new graphic representation of the chemical elements is introduced: chevron form quantum chart.
From the original paper:Genetic_code_quantum_analysis
Genetic code, quantum physics and the 3/2 ratioThe analysis of the quantum structure of the five chemical elements composing the coded twenty amino acids and the four coding nucleotides of DNA working in the organization of the genetic code reveals an opposition of their respective constituents in always an arithmetic ratio of value 3/2 according to the parity of the number of their quantum shells.
https://hal.archives-ouvertes.fr
Résumé
L’analyse de la structure quantique des cinq éléments chimiques composant les vingt acides aminés codés et les quatre nucléotides codant d’ADN oeuvrant dans l’organisation du code génétique révèle une opposition de leurs constituants respectifs en toujours un ratio arithmétique de valeur 3/2 selon la parité du nombre de leur couche quantiques. Aussi, l’analyse quantique de l’acide aminé Glycine, plus petit composant des peptides pouvant être confondu en base saturée, révèle les mêmes oppositions arithmétiques de valeur 3/2 de ses composants par la différenciation de ses cinq groupes chimiques opérée selon leur nombre de protons.
L'article complet en français : Code_Génétique_et_Physique_Quantique
1. Introduction
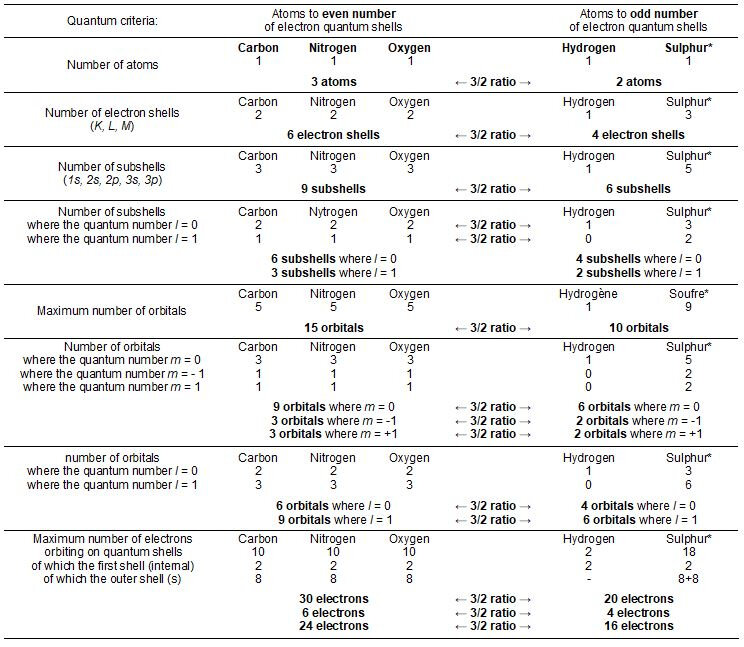
The genetic code is organized into two main entities including a coding structure, DNA (and/or RNA), made up of nucleotides and a coded structure, peptides, chains of amino acids. These two structures each consist of only five different atoms. Thus, Hydrogen, Nitrogen, Carbon, Oxygen and Phosphorus are the only elements of DNA (and RNA) the coding structure of the genetic code. All of the twenty amino acids that make up the peptides, the coded structure, are made up of Hydrogen, Nitrogen, Carbon, Oxygen and Sulphur. These two biological structures therefore each use three atoms with an even number of electron shells (C, N and O) versus two atoms with an odd number of quantum shells (H and P in DNA or H and S in amino acids). These two groups of chemical elements are opposed in various 3/2 value ratios according to almost all of their own quantum criteria.
2. Differentiation of atoms according to the parity of the number of electron shells.
Only five atoms make up the twenty genetically encoded amino acids. These five different atoms distribute their electrons over one, two and three quantum shells. According to these physico-chemical criteria, chart Figure 1, these five atoms are opposed in two groups in a duality of three versus two atoms: Carbon, Nitrogen and Oxygen are with even number of quantum shells; Hydrogen and Sulphur have an odd number of quantum shells. Still in a 3/2 ratio duality, the three atoms with an even number of electron shells total six shells (2 + 2 + 2 = 6 shells) versus four (1 + 3 = 4 shells) for the two atoms with odd number of quantum shells.
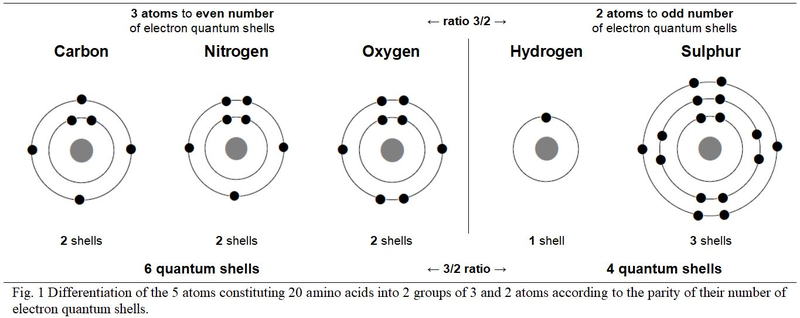
3. Quantum structure
By studying the quantum structure of these five atoms, a multitude of 3/2 ratios is revealed, opposing the three atoms with an even number of electronic shells to the two atoms with an odd number of electronic shells. DNA is also made up of the same five different qualities of atoms except that Phosphorus* replaces Sulphur. However, these last two atoms have the same number of electron shells and the same electronic structure in their saturated state (inside molecules) with the same maximum number of electrons that can orbit their nucleus. This means that the same 3/2 ratio dualities also operate in DNA.
* Phosphorus and Sulphur having the same saturated quantum configuration, these two elements can be confused in some demonstrations.
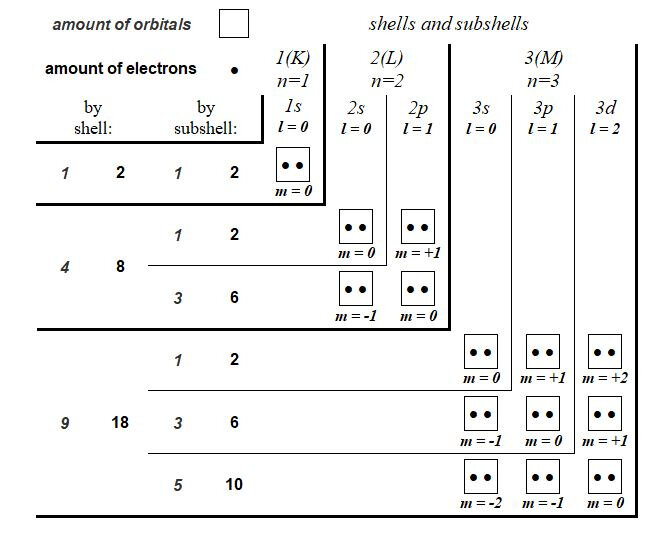
The chart in Figure 2 describes the quantum shells and subshells of electrons of the five atoms constituting the twenty amino acids as well as those of the Phosphorus for DNA. Also detailed are the values of the three quantum numbers n, l and m** as well as the numbers of orbitals. The description of the atoms is that in their saturated state, that is to say with their full electron shells such as they are inside amino acids or nucleotides (DNA).
** Here it is the quantum number mℓ which is the subject of study. For graphic simplification this value is simply noted m in the demonstrations.

The opposition of the values of Carbon, Nitrogen and Oxygen to those of Hydrogen and Sulphur (Phosphorus for nucleotides in DNA), always generates an arithmetic ratio of value 3/2 according to multiple criteria studied.
The table in Figure 3 lists the impressive series of quantum situations in which this remarkable duality takes place between sets of 3x entities versus 2x entities. Thus, the ratio for the numbers of electron subshells (1s, 2s, 2p, 3s, 3p) is 3/2. It is still 3/2 if we detail the subshells of those where the quantum number l = 0 of those where the quantum number l = 1.
Also, the ratio for the numbers of orbitals is 3/2. It is still on 3/2 if we detail the orbitals of those where the quantum number m = 0, of those where the quantum number m = - 1 and those where the quantum number m = 1. This ratio is always 3/2 if we detail the orbitals of those where the quantum number l = 0 of those where the quantum number l = 1. Also, the maximum number of electrons that can orbit inside all of the electronic shells of these two groups of atoms is still in a ratio of 3/2: thirty electrons can orbit inside the electronic shells of Carbon, Nitrogen and Oxygen versus twenty on the electron shells of Hydrogen and Sulphur (Phosphorus for DNA bases).
For this last criterion, the distinction of the electrons which can orbit either on the first internal shell (2 electrons for each of the five atoms) or on the set of the other (external) shells always opposes the different values in ratios 3/2: 6 versus 4 electrons for the inner shell and 24 versus 16 for the other shells.

Thus, fourteen different quantum criteria oppose, in a duality of ratio 3/2, the five atoms constituting the twenty amino acids (and also constituting the four DNA bases with the Phosphorus in place of Sulphur). The fact that the genetic code is organized only with these five different atoms in this duality is therefore not random. The perfect complementarity of the quantum characteristics of Hydrogen and Sulphur (Phosphorus in DNA) is particularly remarkable. These last two atoms have indeed very different quantum characteristics (in contrast to Carbon, Nitrogen and Oxygen with common characteristics) which however complement each other perfectly to always oppose in a 3/2 ratio to three other atoms, constituents of amino acids (and DNA bases). For example, Sulphur has a maximum number of nine orbitals versus only one for Hydrogen. These two very different values nevertheless complement each other (10 orbitals) to oppose in a duality of ratio 3/2 to the three times five quantum orbitals of Carbon, Nitrogen and Oxygen (15 orbitals).
Thus, the 3/2 ratio is revealed at the bottomest of the subatomic structure of the constituents of the twenty amino acids that are on the one hand the three atoms of Carbon, Nitrogen and Oxygen and on the other hand the two atoms of Hydrogen and Sulphur. It is therefore remarkable to note that these same phenomena are found in DNA, another mechanical component of the genetic code, where the quantum properties of the Phosphorus mimic those of Sulphur.
Also, Figure 4, these six atoms constituting the entire mechanism of the genetic code therefore oppose three to three depending on the parity of their number of electron shells. In a ratio of 3/2, the Hydrogen - Phosphorus - Sulphur group totals 63 (3 times 21) nucleons versus 42 (2 times 21) for the Carbon - Nitrogen - Oxygen group. These same two groups are inversely opposed in the 3/2 ratio with respectively nine valences for C, N and O versus six valences for H, P and S.

4 Quantum analysis
4.1 New quantum chart
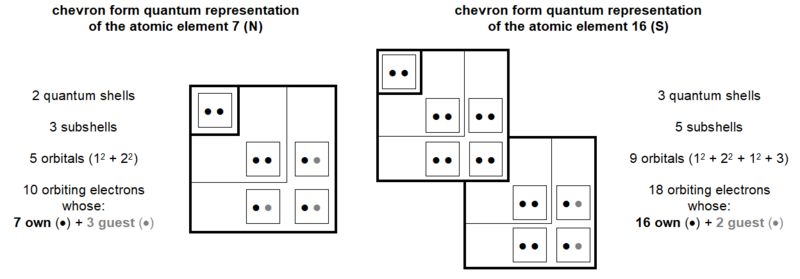
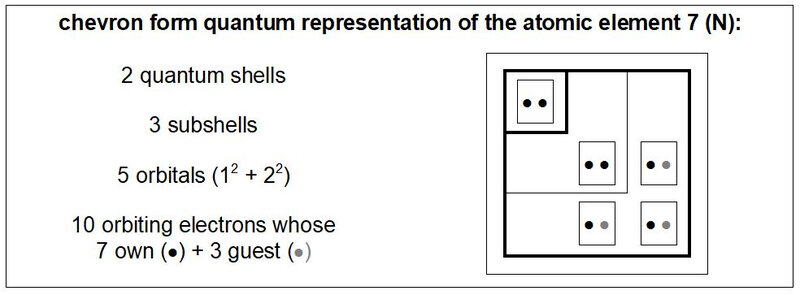
This quantum study of the genetic code is an opportunity to propose a new type of table describing the quantum organization of atoms. In this chart, illustrated in Figure 5, the different quantum shells and subshells are presented in the form of chevrons. At the top end of each rafter are indicated the names of the different shells and subsells; at the left end of these chevrons, the numbers of orbitals and electrons of these different shells and quantum subshells are indicated. At each chevron vertex is the orbital where the quantum number m = 0. The orbitals with positive quantum number m are progressively positioned towards the top of these chevron vertices and the orbitals with negative quantum number m are progressively positioned towards the outside left of these chevron vertices.

In the appendix, the same type of table is presented describing the quantum organization of the shells and subshells up to the 5th shell (O) and 15th subshell (5g). This innovative presentation, more explicit in describing the quantum structure of the atomic elements, will be used in various tables of this quantum study of the constituents of the genetic code.
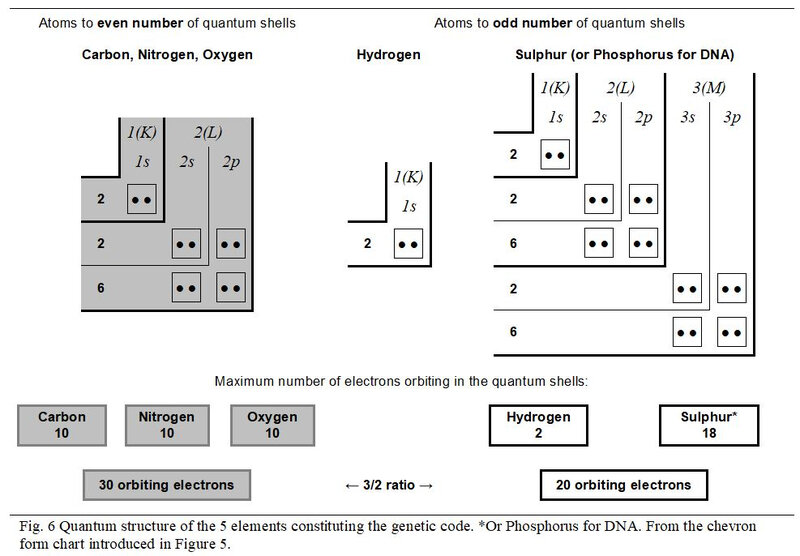
4.2 Quantum structure of atoms
Figure 6 illustrates the quantum structure of the five atoms working in the genetic code. As stated above, the Phosphorus, working in DNA and the Sulphur, involved in peptides, are confused in this analysis. Also, the three atoms Carbon, Nitrogen and Oxygen with even number of quantum shells present the same quantum configuration in their saturated state.
As already introduced in Figure 3, it appears more explicitly in this type of chevron form chart that, in a 3/2 value ratio, the 30 electrons (10 + 10 + 10) orbiting in the three atoms with an even number of quantum shells oppose the 20 electrons (2 + 18) orbiting in the two chemical elements with an odd number of quantum shells.
4.3 Azimuthal quantum number
Figure 7 details the distribution of electrons according to the value of the azimuthal quantum number. It appears that according to this criterion and the parity of the number of quantum shells, the distribution of the electrons of these three and two elements is organized into numerous ratios of 3/2 value including ratios transcendent according to the criteria considered. This arithmetic transcendence is directly related to the remarkable identity (a + b)2 = a2 + 2ab + b2 where a and b have the respective values 3 and 2. This relationship to the remarkable identity which operates in several of the next tables is illustrated and explained in Chapter 4.7.

4.5 Magnetic quantum number
Illustrated in figure 8, the distinction between electrons with a magnetic number m = 0 and those with a magnetic number m = -1 or m = +1 generates exactly the same transcendent arithmetic ratios of 3/2 value. It is essential to emphasize that despite different individual values for the elements H and S, the same global values are found in these counts from two different quantum criteria: the azimuthal quantum number (l) and the magnetic number (m).

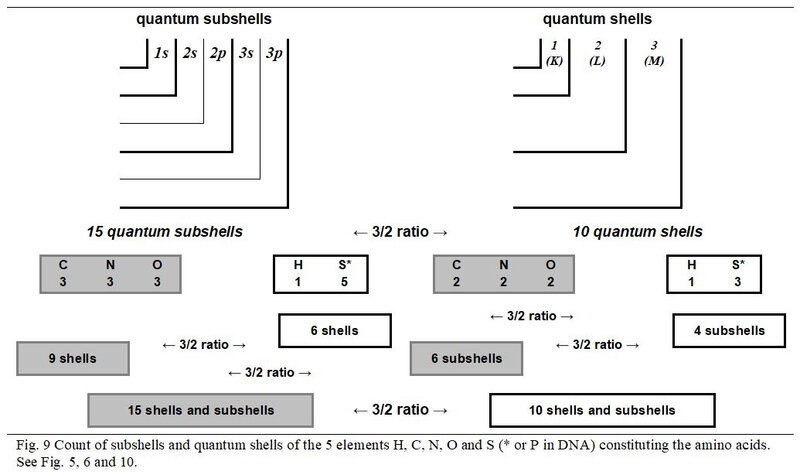
4.6 Quantum shells
As illustrated in Figure. 9, the individual number of subshells of the three chemical elements C, N and O (with even number of shells) is equal to 3/2 of their number of quantum shells. The values of these same ratios are to 1/1 for Hydrogen and 5/3 to Sulphur. However, the global values of these two elements with an odd number of shells complement each other perfectly to also generate a 3/2 ratio between their number of subshells and shells but also with the global values of the three atoms with an even number of quantum shells.
4.7 Remarkable identity
Thus, these various ratios opposing the subshells and shells and transversely, the two categories of atoms previously defined according to the parity of their number of quantum shells, are organized in the remarkable identity (a + b)2 = a2 + 2ab + b2 where a and b have the respective values 3 and 2. Figure 10 explains this arithmetic organization operating in the quantum structure of the five elements working within the genetic code.

Thus, the quantity of subshells in C, N and O corresponds to the value a2 of the remarkable identity and the quantity of subshells in H and S corresponds to the value ab. The quantity of quantum shells in C, N and O also corresponds to the value ab and that in H and S corresponds to the value b2. These different values therefore transcend into these equal ratios:
(a2/ab) = (ab/b2) = (a2+ab)/(ab+b2)
(32/6) = (6/22) = (32+6)/(6+22)
(9/6) = (6/4) = (15)/(10)
As it was previously revealed, this remarkable identity therefore also operates in the counts of electrons according to their azimuthal quantum number (Figure 8) and according to their magnetic number (Figure 9). In these electron counts, the values are just double and, for a and b at the root values 3 and 2, the respective and transcendent values are equal to:
2a2→ 2ab → 2ab → 2b2
18 → 12 → 12 → 8
5 Anatomy of Glycine
Within the mechanism of the genetic code and therefore among the twenty amino acids, Glycine is distinguished by its absence of radical. Its radical is reduced to a simple hydrogen atom which in a way simply closes the "base" structure common to each amino acid. The quantum study of this glycined base, identifying with Glycine, reveals singular arithmetic arrangements of its different components.
5.1 Modules of Petoukhov
The notion of modules is an original system proposed by Sergei Petoukhov [1;2] to describe the structure of biological molecules. In the appendix is introduced this concept of modular structure and detailed two sets of amino acids with the number of protons equal or not equal to eight times their respective number of Petoukhov modules.
5.2 Detailed structure of Glycine
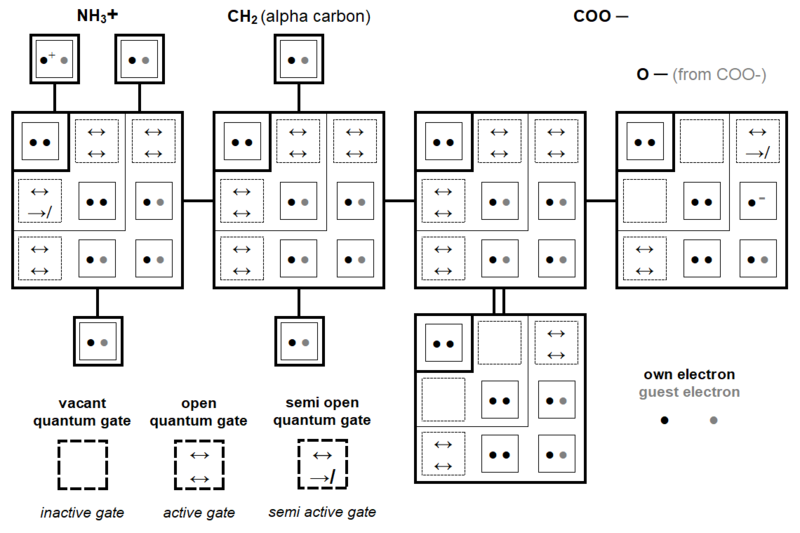
Figure 11 describes the structure of Glycine (or saturated base called glycined base) according to many criteria including its chemical composition, modular, but also atomic. It turns out that Glycine consists of 40 protons, either 5x protons or (3 + 2)x protons. This glycined base also consists of 5 groups or modules, i.e. (3 + 2)x chemical groups. In Glycine, the number of protons is therefore an exact multiple of 8 (5 times 8 protons) and it turns out that the average number of protons per chemical group (or Petoukhov module) is therefore 8. For two groups ( CH2 and O), the amount of protons is exactly 8 whereas for the other three groups, these proton amounts are 9 or 6 (NH2 → 9, OH → 9 and C → 6).
The differentiation of these two types of modules, made up or not made up of 8 protons reveals a multitude of oppositions of the different natures of the components of Glycine (glycined base) in always an arithmetical ratio of 3/2 value. As described in the appendix, the multiplicity of protons/modules within an 8/1 ratio of amino acids is not random, but concerns exactly 50% of the twenty amino acids used in the genetic code, i.e. 10 amino acids out of 20.

Glycine is made up of a multitude of entities whose numbers are all multiples of five. Thus the glycined base consists of five modules, two times five atoms, five of which have one electron shell (H) and five at two shells (C, N and O). Also Glycine consists of 5 times 15 nucleons (75) including 5 times 7 (35) neutrons and 5 times 8 (40) protons. The covalent bonds between these different components are also in numbers which are multiple of 5.

Also, it therefore appears, Figures 11 and 12, that the different constituents of Glycine, always 5x in number, are always at 3 same x entities in the set of three modules (chemical groups) with number of protons not equal to 8 and always of amount at 2 same x entities in the set of two modules whose number of protons is equal to 8.
5.3 Quantum analysis of the glycined base
In the next Figures 13 and 17, the detailed quantum structure of Glycine (shown as glycined base) is illustrated. This graphic representation, more explicit than a classic version, is inspired by the concept of chart in chevron introduced in Chapter 4.
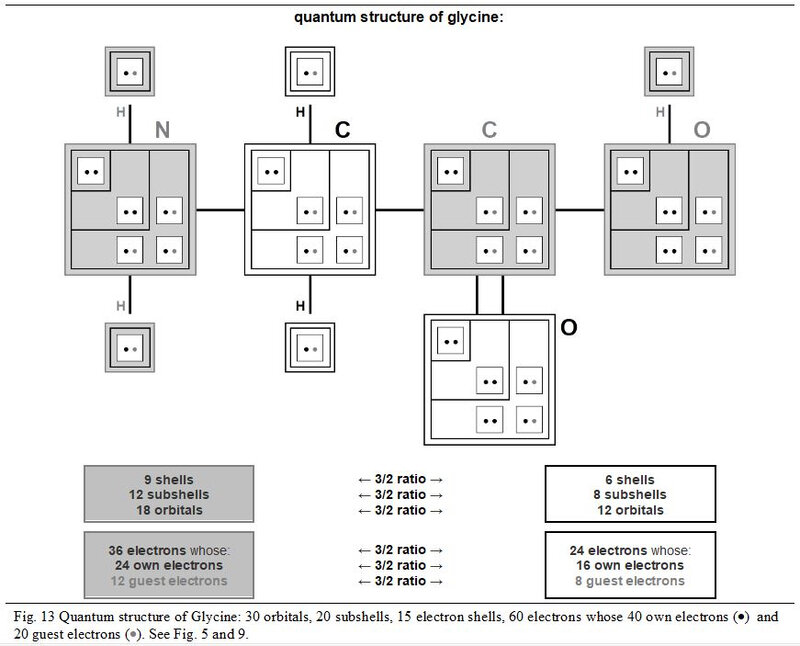
In its quantum structure, Glycine (glycined base) is therefore also always made up of 5x entities. Its ten atoms total 15 (5x → x = 3) quantum shells and 20 (5x → x = 4) electron subshells. These 20 subshells total 30 (5x → x = 6) orbitals where 60 (5x → x = 12) electrons can evolve, including 40 (5x → x = 8) individually own to these ten atoms and 20 (5x → x = 4) covalent electrons (20 shared electrons).
5.3.1 Orbitals, shells, and quantum subshells
Depending on whether they are in the three modules with a number of protons not equal to 8 or in the two with a number of protons equal to 8, these various entities are always with the respective numbers of 3x and 2x. Thus, in these two groups of modules we can oppose, in a ratio of 3/2, 18 orbitals to 12 others, 9 quantum shells to 6 others and 12 subshells to 8 other subshells.
Also, as illustrated in Figure 14, the values of the orbitals and of the quantum subshells oppose in transcendent ratios of value 3/2. So more, this arithmetic transcendence is organized from the remarkable identity (a + b)2 = a2 + 2ab + b2 where a and b have the respective values 3 and 2.
In these counts, the respective and transcendent values are equal to two times the root values of this identity, that is:
2a2→ 2ab → 2ab → 2b2
18 → 12 → 12 → 8
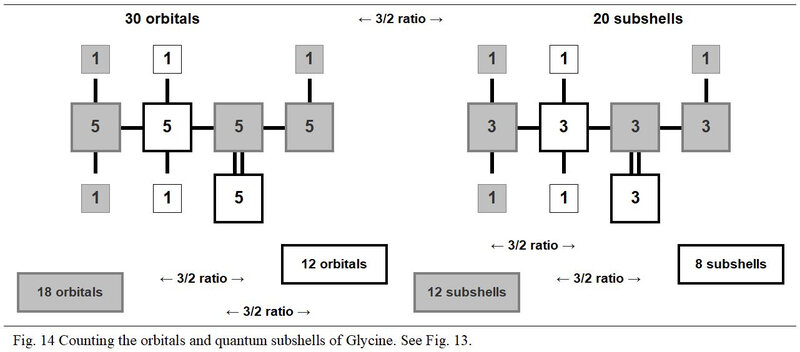
5.3.2 Orbiting electrons and own electrons
As illustrated in Figure 15, depending on whether they are specific to each atom or orbiting (own + invited), and according to their membership of one or the other type of modules (chemical groups previously defined), the electrons of Glycine s oppose in transcendent ratios on 3/2 value. Also, this arithmetic transcendence is organized from the remarkable (a + b)2 = a2 + 2ab + b2 where a and b are to respective values 3 and 2.

In these counts, the respective and transcendent values are equal to four times the root values of this identity, that is:
4a2→ 4ab → 4ab → 4b2
36 → 24 → 24 → 16
5.3.2.1 Electric charges and 3/2 ratio
Since the numbers of clean electrons correspond to those of the numbers of protons, the negative and positive electric charges are therefore also opposed in a ratio of value 3/2 with, for the whole of Glycine, 60 electrons (orbiting) charge negative (-e) versus 40 protons of positive charge (e). The same opposition of electric charges is observed in the two groups of modules previously defined with, for one and the other group, 36 negative charges versus 24 positive and 24 negative charges versus 16 positive charges.
5.3.3 Own electrons and electron sharing
In Glycine, the number of semi-full orbitals is double the number of full orbitals. Also, as illustrated in Figure 16, the distribution of these two types of orbital is organized in 3/2 value ratios between the two groups of modules previously defined according to their proton number equal or not equal to 8.

5.3.4 Jumps in quantum shells
Figure 17 describes, for each atom of Glycine, the amplitude of subshell jumps of electrons which are shared with another atom. Among the 20 shared electrons, it turns out that 10 change (jump) subshell level and 10 do not change level (see also Figure 20).

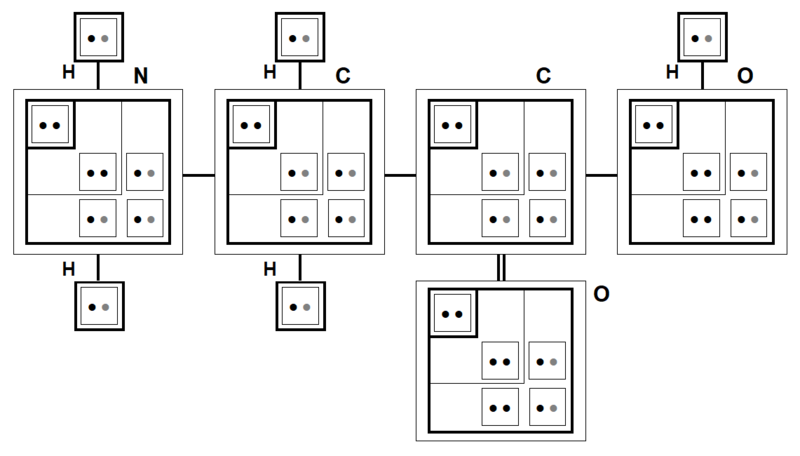
For example (Figure 18), in the NH2 group (module), the own electron of the Hydrogen atom evolves on its original level 1 subshell and on the level 3 subshell of the Nitrogen atom.

5.3.4.1 Jumps and levels of quantum subshells
As it appears Figures 17 and 19, the counting of these subshell jumps always registers in a ratio of 3/2 according to the membership of the electrons to one or the other of the two groups of modules (chemical groups) differentiated according to their number of protons. Also, for each of the shared electrons, the distribution of the cumulative levels of the subshells where these electrons orbit are still organized in a 3/2 value ratio according to these same criteria of differentiation of the modules considered.

5.3.4.2 Electrons shared and subshells
As shown in Figure 20 and previously Figure 17, in their covalent migration, 50% of the shared electrons change of subshell level and 50% are found on the same quantum level. The count of these two types of migration in one and the other group of modules previously defined according to their number of protons is written in arithmetic ratios of value 3/2 with, for each type of migration, six electrons versus four.

5.4 Bonds (valences) and modules
According to the concept of modular structure proposed by Sergei Petoukhov, concept introduced in Chapter 5.1 and detailed in the appendix, it is possible to differentiate two types of covalent bonds:
- the module↔module bonds operating between two non-hydrogen atoms,
bonds which can be qualified as master–master,
- the module↔Hydrogen bonds operating between a Hydrogen and a non-hydrogen atom,
bonds which can be qualified as master–satellite.
As demonstrated in Figure 21 and more explicitly in Figures 13 and 17 where the modular and quantum structures of the atoms are illustrated, the cumulative numbers of master-master bonds and of master-satellite bonds are identical in Glycine (glycined base), i.e. ten bonds (cumulated per atom) for these two categories of covalent bonds.
Also, the distribution of these two types of bonds is organized into 3/2 value ratios between the two groups of modules previously differentiated according to their number of protons which can be equal or not equal to 8.

5.5 Radius and electronegativity
The study of the different main physical values of the chemical elements constituting Glycine* and listed in the table in Figure 22 reveals some like arithmetic phenomena opposing the three modules with a number of protons not equal to 8 to the two with number of protons equal to 8.

5.5.1 Atomic radii
As illustrated in Figure 23, the cumulation of the individual values of the covalent radii, the atomic radii and the Van der Waals radii of the chemical elements constituting the Glycine also register in arithmetic ratios of 3/2 value by the opposition of the two groups of modules previously defined according to their number of protons.
These ratios are not all exactly equal to the 3/2 arithmetic value but approach more than 99%. These differences are justified by the lack of precision (rounding of values) of the source data.
*Note: the values considered are those of the elements in their primordial state.

5.5.2 Electronegativity
As illustrated in Figure 24, the cumulative individual values of the electronegativity of the chemical elements constituting Glycine also fall into arithmetic ratios of 3/2 by the opposition of the two groups of modules previously defined according to their number of protons. This is true from the Allred scale, from the Pauling scale and also from the Mulliken scale with slight oscillations of the ideal ratio of 3/2.
Again, these ratios approach at more than 99% of the ideal 3/2 value ratio and these differences are justified by the variability and relative imprecision of the raw source data on these different scales.

Thus, although different individually, these different physical values of atomic dimension and electronegativity are nevertheless and always organized in arithmetic ratios of value 3/2 by the opposition of the modules (chemical groups) with equal or unequal proton number to the value 8.
6 Quantum analysis of Methionine
We have just demonstrated that the quantum study of Glycine reveals an organization of its components in various arithmetic arrangements, mainly the 3/2 ratio. With less amplitude, but still very significantly, another proteinogenic amino acid, quantically organizes itself in similar arrangements. This amino acid is not just any, it is Methionine, initiating (and being the only one in this case) any peptide chain.
Figure 25 describes the quantum structure of Methionine where a maximum of 120 electrons can orbit, a value double that of Glycine and also equal to 5x.

Fig. 25 Quantum structure of proteinogenic amino acid Methionine. See Fig. 26 and 27 also.
6.1 All orbiting electrons versus own electrons
In the next Figures 26 and 27 is counting some quantum values of Methionine: orbiting and own electrons, orbitals and subshells.

Exactly like for Glycine (See Figure 15, Chapter 5.3.2), as illustrated Figure 26, the quantity of electrons that can evolve on the orbitals of Methionine (120 electrons) and the quantity of electrons which are own to this amino acid (80 electrons) are opposed in a ratio of value 3/2.
6.2 Orbital amount versus subshell amount
Exactly like for Glycine (See Figure 14, Chapter 5.3.1), as illustrated Figure 27, the quantity of atomic orbitals of Methionine (60 orbitals) and the quantity of subshells inside atoms of this amino acid (40 subshells) are opposed in a ratio of value 3/2.

7 Quantum configuration of carbon
Among the five chemical elements constituting proteinogenic amino acids and more broadly within the various organic molecules, it is recognized that carbon plays a primordial role. The following observation is therefore not timely.
7.1 Carbon and 3/2 ratio
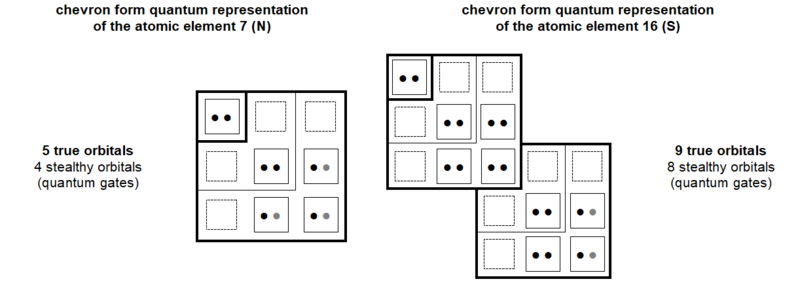
As illustrated in Figure 28, since its excited state then becoming saturated, carbon can therefore total ten electrons on its orbitals, including six own electrons and four guest. These two sets of electrons are thus opposed in a 3/2 value ratio. Of the five chemical elements that make up amino acids (H C N O S, see Figures 2 and 13) or DNA/RNA (H C N O P), only Carbon has this 3/2 ratio between own and guest electrons.

8 The first ten chemical elements
From the viewpoint of filling the orbitals, the first ten chemical elements deserves special development. Thus, Figure 29, the chemical element of number 10, is the one which completely fills the first three quantum subshells.
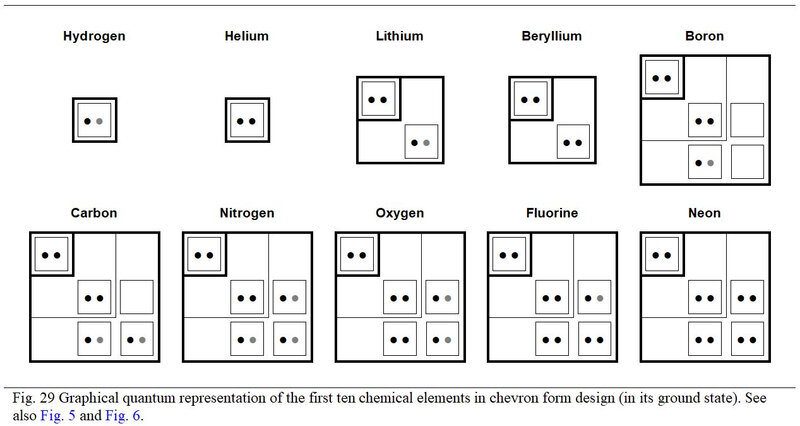
Also, the own values of the atomic numbers of these first ten elements generate singular arithmetic phenomena to whether these elements are inorganic or organic. Moreover, for these first ten chemical elements, the distribution in detail of their electrons according to the parity of the rank of the quantum subshells is particular. It is necessary to highlight this before concluding this quantum study of the genetic code.
8.1 Inorganic versus organic elements
It turns out that among the first ten chemical elements, in a 3/2 ratio opposition, six are not used in the genetic code (Helium, Lithium, Beryllium, Boron, Fluorine and Neon) while four elements (Hydrogen, Carbon, Nitrogen and Oxygen) are used. More generally, we can therefore say that six of these ten chemical elements versus four are not elements of living matter since they do not compose organic molecules.
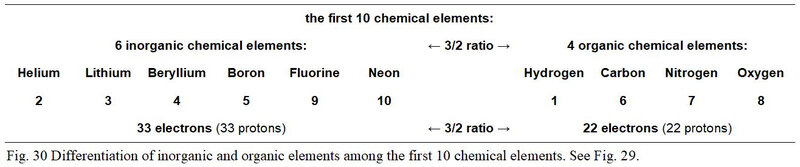
Also, as it appears in Figure 30, the sums of the own electrons of these two groups of six and four chemical elements are as well opposed in an arithmetic ratio of value 3/2. It turns out that the group of six inorganic elements totals 33 electrons while that of the four organic elements totals 22.
8.2 Odd subshells versus even subshells
Top Figure 31 shows that in a ratio of 3/2 value, the first 6 inorganic chemical elements total 24 electrons into their odd subshells (1s and 2p) versus 16 electrons for the first 4 organic chemical elements.
Down Figure 31 shows that in a ratio of 3/2 value, the first 6 inorganic chemical elements total 9 electrons into their even subshells (2s) versus 6 electrons for the first 4 organic chemical elements.
All this is observed by considering these elements in their ground state as described in Figure 29.

Discussion et Conclusion
Analysis of the quantum organization of the chemical elements working in the constituents of the genetic code reveals a systematic opposition of their different components in a 3/2 value ratio depending on whether these atoms have an even or odd number of quantum shells. The multitude of these singular arithmetic arrangements, always identical in their final ratios, prohibits the idea of any random interaction of these different constituents.
These arithmetic configurations are of a highly structured level, often even organized around the remarkable identity (a + b)2 = a2 + 2ab + b2 where a and b have precisely the respective values 3 and 2. The arithmetic mechanics arising of this identity thus allows the different values considered to be organized and opposed in a triple ratio of 3/2 transcendent values and in arithmetic form: (a2/ab) = (ab/b2) = (a2+ab)/(ab+b2).
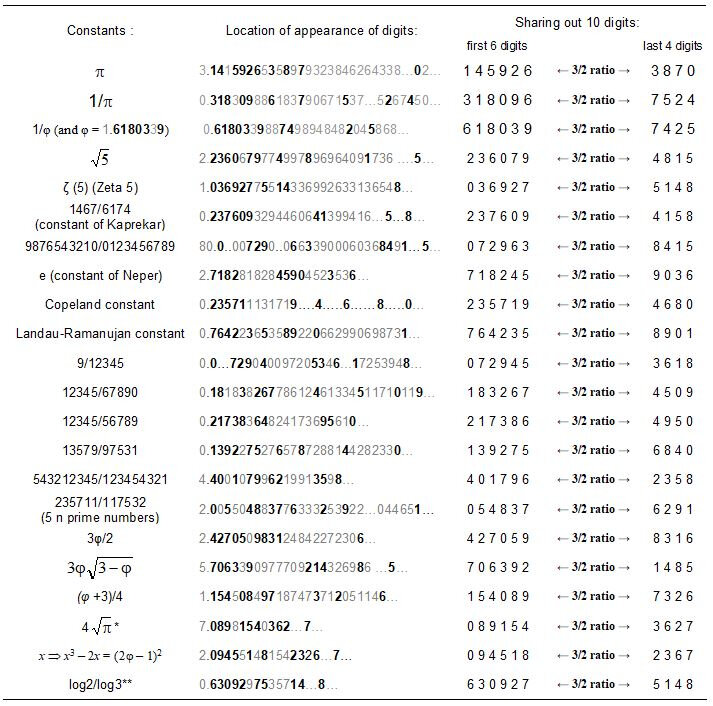
In another paper [3] and yet in a very different scientific field, that of the study of whole numbers, we also observed the organization of entities in this 3/2 ratio and also in this same remarkable identity. This therefore supports the idea of a universality of these arithmetic phenomena.
As highlighted in some of his various works including those referenced in [1] and [2], Sergei Petoukhov draws attention to the organization of the components of the genetic code around the values 3 and 2. For example , and this is of great importance, there are two types of DNA base associations, one with three hydrogen bonds between the bases Guanine and Cytosine and the other with two hydrogen bonds between the bases Adenine and Thymine.
The fact that this 3/2 arithmetic ratio also operates between the components of Glycine, the primary amino acid identified to a glycined base, supports the idea of an arithmetically non-random organization of the mechanics of the genetic code. The latest investigations studying the different values of radii and electronegativity of the components of this glycined base greatly reinforce this analysis.
In this organization of living matter, its modular structure, the multiplicity (or not multiplicity) protons/modules and more broadly the multiplicity (or not multiplicity) of the numbers of protons of the twenty amino acids by the value 8 appear to be factors to consider with the utmost care. Also, we suggest, without discussing it further here, that this criterion of multiplicity by 8 of the number of protons in amino acids is related to the byte rule, another quantum constraint operating in the chemical elements used in these amino acids.
To conclude this quantum study of the components of living matter working in the organization of the genetic code, we advance the idea that this matter known as "living" is only the prolongation of a general organization of matter since its atomic structure towards its molecular structure. Indeed, as it is revealed in this study, the elements working within living matter are not randomly organized according to arithmetic criteria which depend on their primordial quantum structure.
This 3/2 fractional value arithmetic ratio is very similar to the fractional values of the electric charges of different quarks, which are ratios of whole numbers (2/3 and -1/3). Thus these phenomena, operating in the most complex organization of matter, depend on its most basic structure. By the amplitude of the phenomena presented here, it is therefore not possible to imagine a non-relation between this primary structure of matter and its highly organized structure as it appears in the structural mechanics of the genetic code.
Appendix
A1. Quantum chart in chevron form
A1.1 New quantum chart
Figure 32 shows the new chevron form quantum table of the first 15 electronic shells. This graphic concept is introduced in Chapter 4.1 and illustrated in Figure 5. We suggest that this new graphic type be favoured for the description of the quantum organization of the different chemical elements.
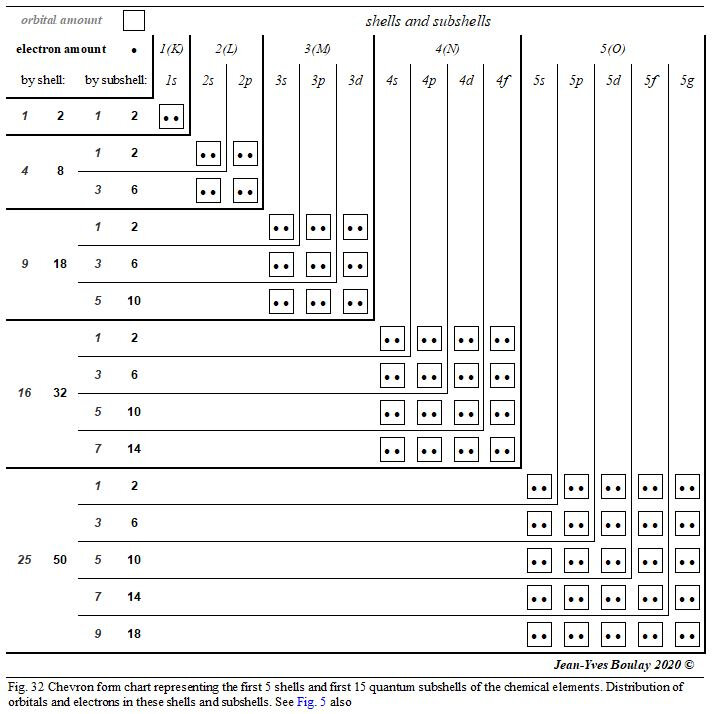
A1.2 Quantum chart depiction
In this table, the different quantum shells and subshells are presented in the form of chevrons. At the top end of each rafter are indicated the names of the different shells and subshells; at the left end of these chevrons, the numbers of orbits and electrons of these different shells and quantum subshells are indicated. At each chevron vertex is the orbital where the quantum number m = 0. The orbitals with positive quantum number m are progressively positioned towards the top of these chevron vertices and the orbitals with negative quantum number m are progressively positioned towards the outside left of these chevron vertices.
This new graphic design is more explicit in describing the quantum structure of atomic elements than any other usual linear chart. Very visually, this chevron configuration clearly highlights the arithmetic progression of the orbital numbers of the different quantum shells in square powers of the level of these electronic shells:
- 1st shell → 12 = 1 orbital,
- 2nd shell → 22 = 4 orbitals,
- 3rd shell → 32 = 9 orbitals,
- 4th shell → 42 = 16 orbitals, etc.
A.1.3 Atom quantum chart
In this table, and more generally in this quantum study of the chemical elements, constituents of the genetic code, the electronic spin is not detailed (by ascending or descending arrows). In return, it is the migratory or non-migratory nature of the electrons that is about development. Thus, for example, the representation of the nitrogen atom such as that illustrated in Figure 33 (and in various other figures of this quantum study) is favoured.

A.2 Number of protons and modules of the twenty amino acids
A.2.1 Modules of Petoukhov
The notion of module is an original system proposed by Sergei Petoukhov [1 and 2] to describe the structure of biological molecules. This system represents each non-hydrogen atom by the number of protons in its nucleus. For example, the nitrogen atom is represented by the number 7. If one or more hydrogen atoms are joined to a non-hydrogen atom, the number of hydrogen protons is added to the number of atom protons of non-hydrogen.

Thus, such a group made up of a non-hydrogen atom and its adjoining satellite atoms of Hydrogen is defined by the total sum of protons of this group and this set is called "module". Figure 34 illustrates this modular concept applied to amino acids.
For example, the amide group NH2 forms a module: it is denoted by the number 9 which is the sum of 7 protons of one nitrogen atom and 2 protons of two satellite atoms of Hydrogen. Sergei Petoukhov provides [1 and 2] modules from 6 to 9 protons according to the configurations. Thus according to this system, the Sulphur atoms (present in Met and Cys) break down conventionally into two modules of 8 protons (or 9 protons if associated with a Hydrogen in Cys).
A.2.2 Protons/modules multiplicity
The genetic code is organized only with 20 amino acids. Figure 35 isolates amino acids with a number of protons multiple of the number of modules from those with a number of protons not multiple of the number of modules. It turns out that these two groups are of equal size since they each consist of 10 entities.

It is remarkable to note that the respective number of protons of each of the ten amino acids of the first group, that whose number of protons is multiple of the number of modules, is always equal to eight times the number of modules.
Also, for this first group of ten amino acids, the total number of modules is equal to 80, so an average of 8 modules into each amino acid. Thus, the total number of protons in this group is therefore equal to 640 protons, an average of 64 (82) protons by amino acid. Also, for each of the ten other amino acids, the proton/module ratio never represents an integer but a fraction different from x/1 (x being an integer).
A.2.3 Other multiplicities
The number of proteinogenic amino acids is 20 and this number is not by chance. It is equal to 5x (5x → x = 4). This value therefore allows the formation of a 3/2 ratio since this number is therefore equal to 3x + 2x (3x + 2x → x = 4).
As described in the left part of Figure 36, it turns out that two other amino acids have a number of protons multiple of 8 but not multiple of their respective number of modules: Phe with 88 protons for 12 modules and Tyr with 96 protons for 13 modules. Thus, by these multiplicity criteria, in a ratio of value 3/2 are opposed twelve amino acids with number of protons multiple of 8 versus eight amino acids with number of protons not multiple of 8.

Also, as described in the right part of Figure 36, in an inverse ratio of 2/3 value, height amino acids versus twelve have a number of protons multiple of their respective number of chemical groups. As explained above, the nuance between chemical groups and modules is observed for chemical groups composed of a sulphur atom, an atom conventionally made up of two modules (see Figure 27). Thus, the amino acids Cys and Met have an amount of chemical groups which differs to one value in relation to their number of modules.
A.2.4 Three progressive arithmetical ratios
Thus, illustrated in Figure 37, according to three different criteria for the multiplicity of proton numbers, the ratio between the twenty amino acids operating in the genetic code changes from the value 3/2 to the value 1/1 then to the value of 3/2 , inverse to the initial.

All these observations confirm the differentiations made in the chemical, quantum and atomic study of Glycine between the modules (chemical groups) with a proton number equal to 8 and from the modules with a proton number not equal to 8.
References
1. S.V.Petoukhov. Genetic Code and the Ancient Chinese Book of Changes, Symmetry Culture and Science, vol.10. 1999.
2. S.V.Petoukhov. The Bi-periodic Table of Genetic Code and Number of Protons. 2001.
3. Jean-Yves Boulay. The ultimate numbers and the 3/2 ratio. 2020. ⟨hal-02508414v2⟩
4. www.elementschimiques.fr
Jean-Yves BOULAY independent researcher (without affiliation) – FRANCE -
e-mail: jean-yvesboulay@orange.fr
https://orcid.org/0000-0001-5636-2375
Original full text here:Genetic_code_quantum_analysis
L'article complet en Version française :Code_Génétique_et_Physique_Quantique
Jean-Yves BOULAY






































































/https%3A%2F%2Fstorage.canalblog.com%2F27%2F84%2F1688828%2F128119988_o.jpg)
/https%3A%2F%2Fstorage.canalblog.com%2F81%2F46%2F1688828%2F127748506_o.png)
/https%3A%2F%2Fstorage.canalblog.com%2F40%2F49%2F1688828%2F127443252_o.jpg)
/https%3A%2F%2Fstorage.canalblog.com%2F02%2F53%2F1688828%2F127394551_o.jpg)
/https%3A%2F%2Fstorage.canalblog.com%2F63%2F57%2F1688828%2F127284281_o.jpg)
/https%3A%2F%2Fprofilepics.canalblog.com%2Fprofilepics%2F1%2F7%2F1766149.jpg)